The term “impartiality” in the ISO/IEC 17025:2017 standard expresses objectivity meaning that the outcome of calibration and testing activities shall not be influenced by individual actions and circumstances.
- Key Requirements related to Impartiality in ISO 17025 standard
- What should be avoided?
- Step by Step implementation of Impartiality requirements
- Frequently Asked Questions (FAQs)
Key Requirements related to Impartiality in ISO 17025 standard
- Commitment of management toward impartiality
- As a core requirement the standard requires the laboratory to Identify and mitigate all risks that can threaten impartiality.
- These risks shall be monitored on a continuous basis.
- Standard has defined three type of risks that threaten impartiality i.e.
- Risks emerging from laboratory activities and operations.
- Risks emerging from the laboratory’s relationship with other entities including its customers
- Risks emerging from the relationship of employees with the laboratory, its customers, and other stake holders, such as suppliers
- In addition, the standard has defined three cornerstones of laboratory policies and objectives i.e. competence, consistent operations and impartiality. Accreditation bodies evaluates these three key elements in laboratories seeking accreditation.
The objective of including impartiality in the standard is not merely to safeguard the validity of results but it extends to avoiding unwarranted situations or actions by ensuring no conflict of interest and unbiased use of laboratory resources, management structure, and laboratory processes.
What should be avoided?
The objective is to avoid unwarranted situations that can result in conflict of interest, bias, unfairness, prejudice, and nepotism that could ultimately affect laboratory functioning in an ideal manner.
These situations can arise from financial, workplace, commercial, and other pressures.
Example of such conflict of interest are as under
Laboratory Activities & Operations
- Technician bonuses are linked to the quantity of calibration performed resulting in hasty calibration and procedural lapses such as overlooking environmental conditions while performing calibration.
- An Internal auditor overlooks some non-conformities in the calibration procedure because it was prepared under his own supervision, leading to biased reporting.
Laboratory’s relationship with other entities including its customers
- The laboratory’s 60 % revenue is dependent on one customer, leading to cautious reporting of non-conforming results.
- Delay in calibration of client’s equipment to accommodate internal department request, showing bias in resource allocation.
Relationships of employees with the laboratory, its customers, and other stake holders, such as suppliers
- Spouse of the Lab Manager work as a sale rep with a key supplier leading to biased procurement decisions affecting calibration accuracies.
- A Laboratory technician receives a gift or incentive from a customer to fast track or tamper with the results.
Step by Step implementation of Impartiality requirements
- Get commitment from top management
- Create awareness among staff
- Get legally enforceable declarations
- Identify and manage risks to impartiality
- Continuous Monitoring of the risks
Step 1: Get commitment from top management
The standard requires that the top management of the laboratory shall be committed to impartiality. The practical demonstration of this can be through an impartiality policy. This can be done by either making an impartiality statement part of the quality policy or drafting a separate impartiality policy. A template of an impartiality policy is shown below:
Step 2: Inculcate awareness and impartiality culture
Impart awareness to the laboratory staff by conducting awareness sessions, discussing case studies, conducting brainstorming sessions, etc.
Step 3: Get legally enforceable declarations
Make binding for all laboratory personnel to sign an impartiality agreement or undertaking. The agreement shall ensure
- Disclosure of personal relationships
- Disclosure of past associations
- Disclosure of any type of conflict of interest
- Pledge to uphold impartiality while performing laboratory activities
- Disciplinary action in case of impartiality breach
Step 4: Identify and manage risks to impartiality
Risk Identification:
The standard requires the laboratory to identify risks related to all laboratory activities. However, identification of risks related to impartiality has been mentioned separately in the standard hinting at clear emphasis.
Risk identification can be done through brainstorming sessions, interviews, document review or seeking help from experts. Identification does not mean noting down the unwanted events which have already occurred but it also includes assessment of potential risks in your system.
As discussed, earlier the identification of risks shall include risks emerging from laboratory operations, its relationships and relationships of its employees.
All identified risks shall be included in the risk register or risk management plan
Risk Management:
Risk Management includes analyzing and evaluating an identified risk i.e.
- Assessing its probability of occurrence
- Assessing its severity or impact
- Determining the risk score from the above two.
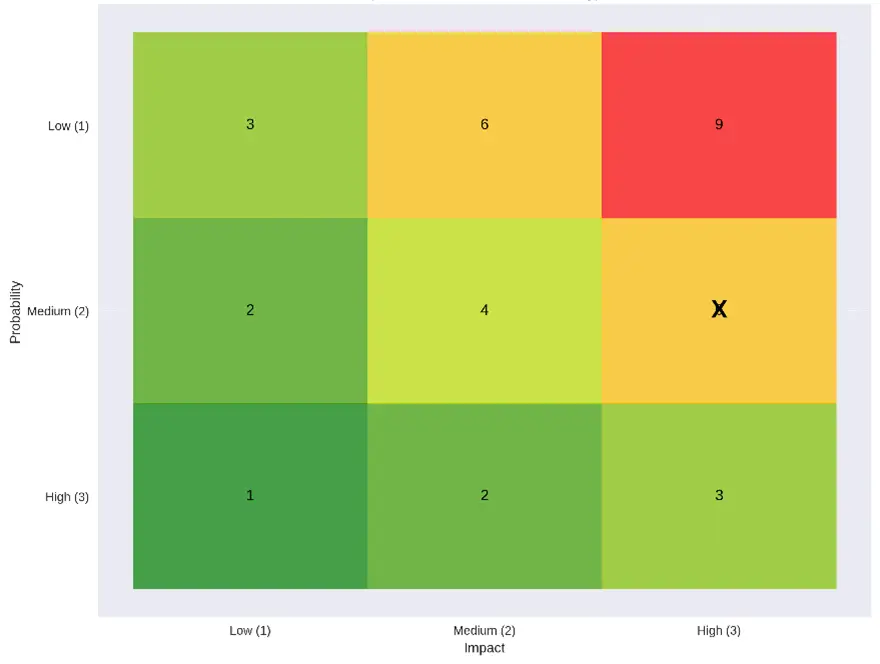
Best way to do it is through impact vs probability matrix shown in figure below.

Example
Let’s take an example of a risk to impartiality in a calibration laboratory
Risk Description
Hasty calibrations and procedural lapses (such as overlooking environmental conditions) by calibration technicians while performing calibration to show a high volume of calibrations performed because the company has linked the performance incentive to the volume of calibrations performed
Risk Likelihood
Incentivizing the technicians based on their productivity and throughput is a common practice in industry. Lab technicians are well trained and professionals. Furthermore, regular audits also reduce the probability of occurrence of such an event, so likelihood of the risk is Medium
Risk Impact
Hasty calibrations and procedural lapses can severely impact the validity of calibration results, consequently harming customer interests. It is a clear noncompliance of impartiality requirements. Hence the impact of risk is High.
Risk level/Score
High (medium Likelihood x high impact). So immediate action shall be taken to mitigate the risk.

We can also quantity the risk score as shown in the color matrix below. Risk score here is 6 i.e
Likelihood (medium) = 2
Impact (high) = 3
Risk Score = 2 x 3 = 6
Mitigation Strategy:
- Revisit the incentives policy and give weightage (50-50 quantity vs quality split) to quality of calibrations as well while distributing incentives.
- Impart training to technicians regarding impartiality requirements and make sure that they have signed an impartiality undertaking.
- Use the risk as an opportunity to improve your process by introducing automation such as the use of data loggers for monitoring environmental conditions.
Step 5: Continuous Monitoring
Most of the risks cannot be fully eliminated and residual risk still exists after mitigation. Continuous monitoring is necessary to ensure that residual risks remain at an acceptable level.
In the example discussed in last section, the residual risk will be medium to low.
Some of the monitoring tools which a lab can deploy are as follows
- Regular review of the risk management plan
- Discussion on impartiality in management review meeting
- Continuous assessment of staff awareness on impartiality
- Detailed assessment of compliance level during internal audit
To conclude, adopting a systematic or step-by-step approach can make your work of implementing impartiality very easy. The approach discussed in this article ensures proactive implementation of impartiality requirements which is in alignment with the emphasis of the standard, i.e. continuous risk evaluation.

